
Elements of this group include: hydrogen, sodium, potassium, and lithium. The oxidation number of elements in group one (alkali metals) of the periodic table is usually +1.Examples of peroxides include hydrogen peroxides, H 2O 2(H-O-O-H).

Except in peroxides, where oxygen has an oxidation number of -1.
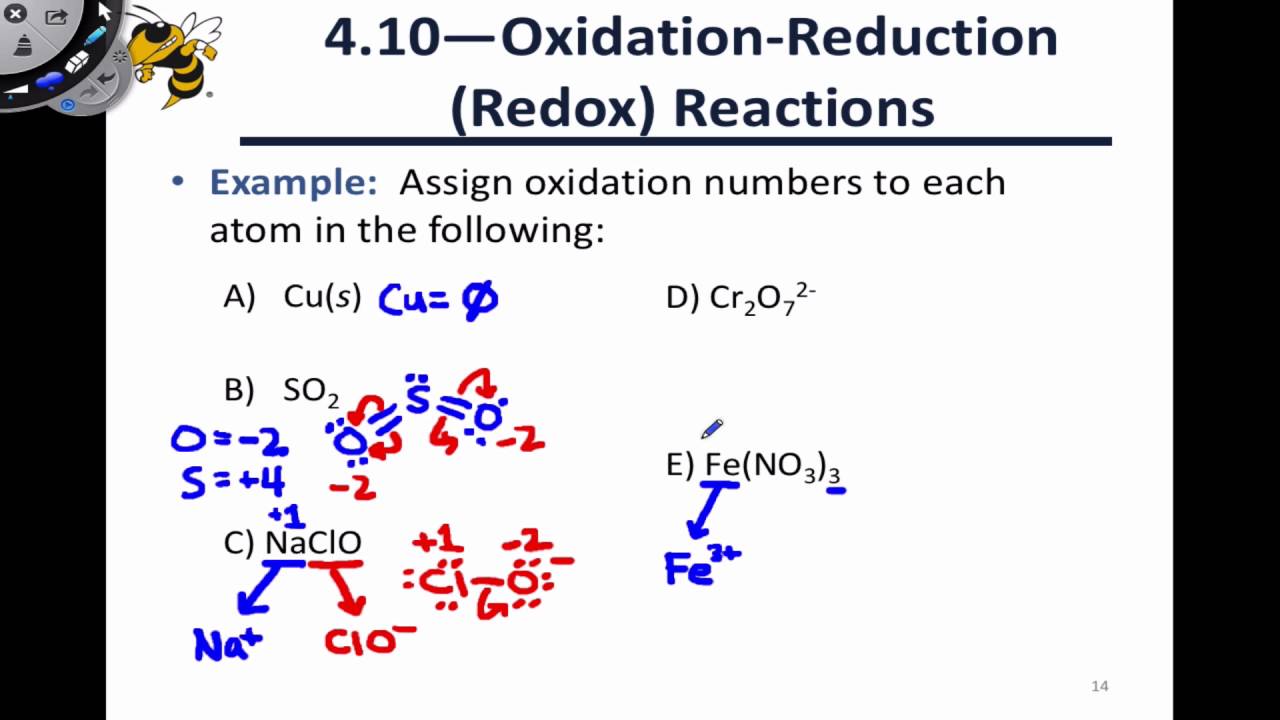
The oxidation number of an oxygen atom in most compounds is -2. Recall that from the electron bookkeeping method, when two atoms of the same element share electrons, you must divide the shared electrons equally between them. Iodine: I 2 Bromine: Br 2 Aluminum: Al Helium: He Iron: Fe.Īs you can see, elements including iodine, bromine, oxygen, chlorine, hydrogen are all diatomic. The oxidation state of an atom in the most abundant form of an element is zero.Īs a result, elements including the following have an oxidation number of zero:. To use this shortcut, we must consider the following rules: This shortcut does not apply to all compounds, but we can use it to assign oxidation numbers to many compounds we encounter in chemistry. Because of this, chemists have devised a shortcut for assigning oxidation numbers. Remembering electronegativity values by heart is difficult and drawing an electron dot diagram does take time. However, it usually requires electronegativity values and an electron dot diagram. 
The oxidation bookkeeping method is a reliable way of determining the oxidation number of an atom. In a polyatomic ion, the sum of the oxidation numbers of all the atoms in the ion must be equal to the charge on the ion.Indirect Addressing with Productivity Suite Software In a neutral atom or molecule, the sum of the oxidation numbers must be 0.







 0 kommentar(er)
0 kommentar(er)
